
RHA® 10 Anni
La nostra tecnologia avanzata, sviluppata attraverso un processo brevettato1 e da una ricerca all'avanguardia, è il cuore dei nostri filler dermici e dei nostri dermocosmetici.
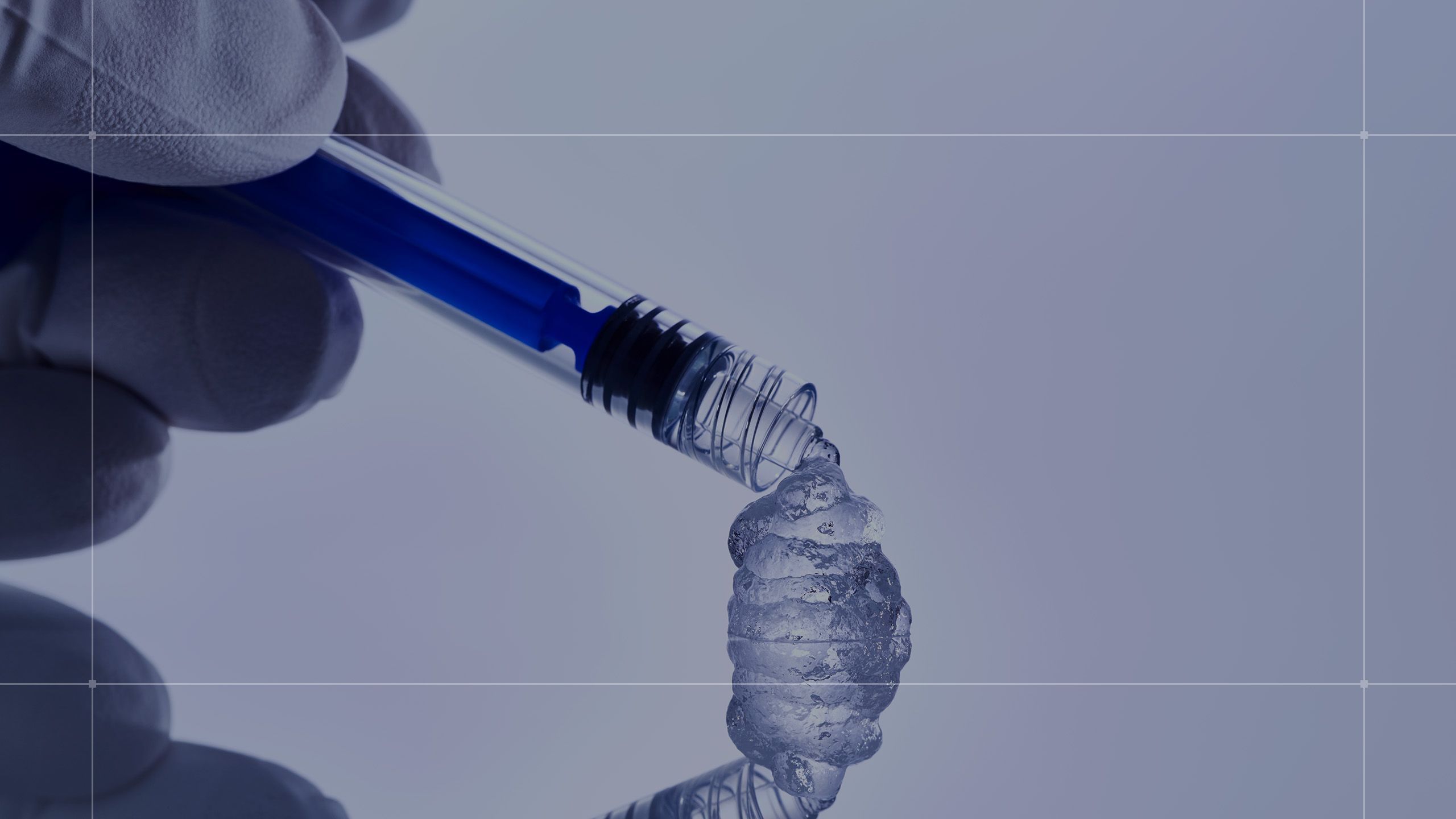
Cosa rende RHA® Unico?
Scopri la scienza di Resilient Hyaluronic Acid® (RHA®) di Teoxane, per risultati dinamici e naturali.2

La Scienza della Bellezza Naturale
La "Preserved Network Technology" di Teoxane è un rivoluzionario processo brevettato di produzione dell’acido ialuronico che non prevede l’impiego del calore3 che ha trasformato la creazione di gel di acido ialuronico.